Configuration is saptical arrangement of atoms present in molecules which is stable in general conditions. There is large difference of energies in these configuration. To interconvert one configuration to another bond fission or bond formations is necessary which is not possible in ordinary conditions. They can be isolated with each other. If different configuration obtained due to presence of chiral carbon. Then they are known as optical isomers and these isomers can be differentiated by putting a prefix D and L or R and S
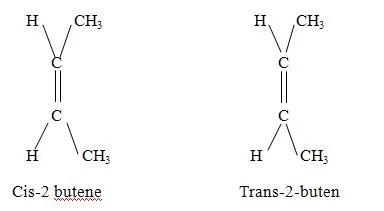
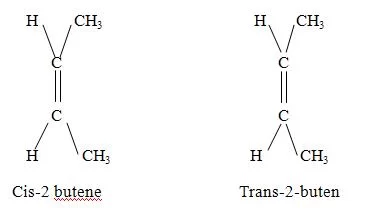
In these two structures the position of H and – OH is opposite. Structure I is not inter convertible tp (II). For this both C-H and C-OH bonds should break and form in opposite positions.
For inter conversion bond fission or bond formation is necessary so they can be isolated. Contrary to this due to free rotation of carbon-carbon single bond different relative positions are formed known as conformations and these relatives structures are known as conformers. Since these different conformers have little energy difference so in general they are inter convertible and they can’t be isolated. For example, ethane have two types of extreme conformations.
(i) Eclipsed conformation
(ii) Staggered conformation
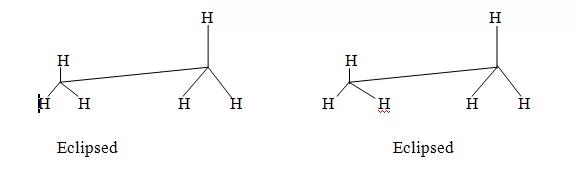
Energy of staggered form is lower than eclipsed form. The different in energy is 28 kcal mol-1.
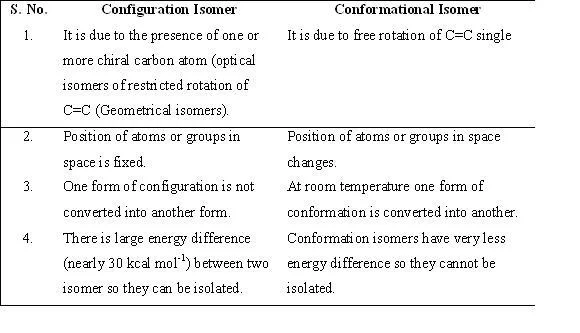
Table : Difference between configuration and conformations.