VSEPR THEORY (Valence sheel electron pair repulsion theory)
• Given by Gillespie and Nyhlom
• It deals with the shape of the moleculeShape of the Molecule : It is the space model that is obtain after the joining the point represents the bonded atoms.
POSTULATES OF VSEPR THEORY:-
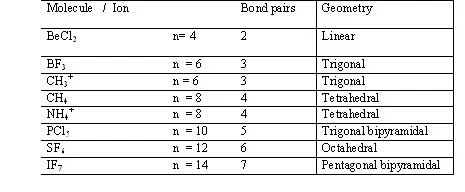
1. If all e(-) pair around the central atom are b.p. (there are no l.p.) than Molecules posseses a regular shape and it’s depend upon the nature of hybridization)
Examples
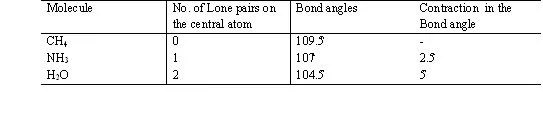
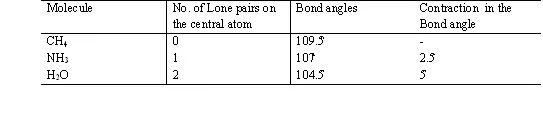
2. If all electron pair around the central atom are not bond pair there are same lone pair also then the molecule posseses a distorted shape because of –
1.p. – l.p. > l.p. – b.p. > b.p. – b.p. repulsion )
3. Bond angle decreases with the increase of electronegativity of the atoms attached to the central atom.
(i) AsI3 (101̊) > AsBr3 (100.5̊) > AsCl3 (98.4̊)
4. If all electron pair around the central atom are bond pair and all surrounding atoms are identical than change in E.N. in the central atom as well as in the surrounding atom does not alter the bond angle :
e.g. CH4 = CCl4 = CBr4
5. Double bond occupy more volume than the single bond: