Cahn – Ingold – Prelog priority sequence can be usefully applied in giving names to such a structure. The group of highest priority, on both the double bonded carbon atom is first chosen. If the two group of highest priority are on the opposite side of the double bond. The alphabet E (from the German word Entagagen meaning opposite) is used for the structure and if the groups of highest priority are on the same side the alphabet Z (from German word, Zussamen meaning together) is used. Thus E stands for opposite side and Z for the same side. For example.
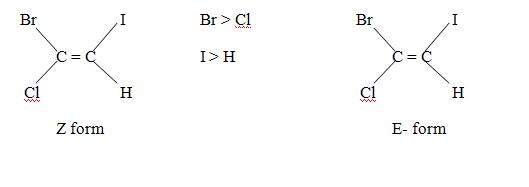
Generally cis isomer is said to be Z form and trans isomer is said to be E forms but due to sequence rule there are some exception. For example, 1,2 dichloro – 1 – bromoethene
Although CI atom present on opposite side but according to sequence rule it is Z form.
Determination of the configuration of geometrical isomers
(i) Physical methods:- Generally trans-isomer had high m.p. and low b.p. than cis isomer.
(ii) Solubility of cis isomer is more. For example, solubility of maleic acid is 3.0 g/100 mL of water at 293 K and solubility of fumaric acid is 0.7 g in 100 mL of water at 293 K
(iii) Dipole moment measurement:- the trans isomers are symmetrical and hence will have zero or low dipole moment as compared to the cis.
μ = 0
(iv) Chemical method:- (method of cyclisation)
(i) In this method geometrical isomers may be obtained from cyclic compounds. If cyclic compound has two groups at cis and trans position than compound obtained will be in the similar position. For example, benzene or p-quinone on oxidation forms unsaturated dicarboxylic acid.
In benzene and p-quinone both hydrogen atoms are in cis position to double bond they will be at cis position in dicaboxylic acids and thus dicarboxylic acid is in cis form and another isomer will be trans (fumaric acid m.p. 575 K).
(ii) By changing geometrical isomer in cyclic compounds: A molecular containing two – COOH groups attached to different carbon atom will form an anhydride. If the groups are on same side i.e. the structure is cis then maleic acid forms anhydride easily and therefore it can be identified as cis compound. In furmaric acid the two – COOH groups are on the opposite side and therefore it is very difficult for them to interact under such conditions in which maleic acid does.
